Give the Full Electron Configuration for Sulfur.
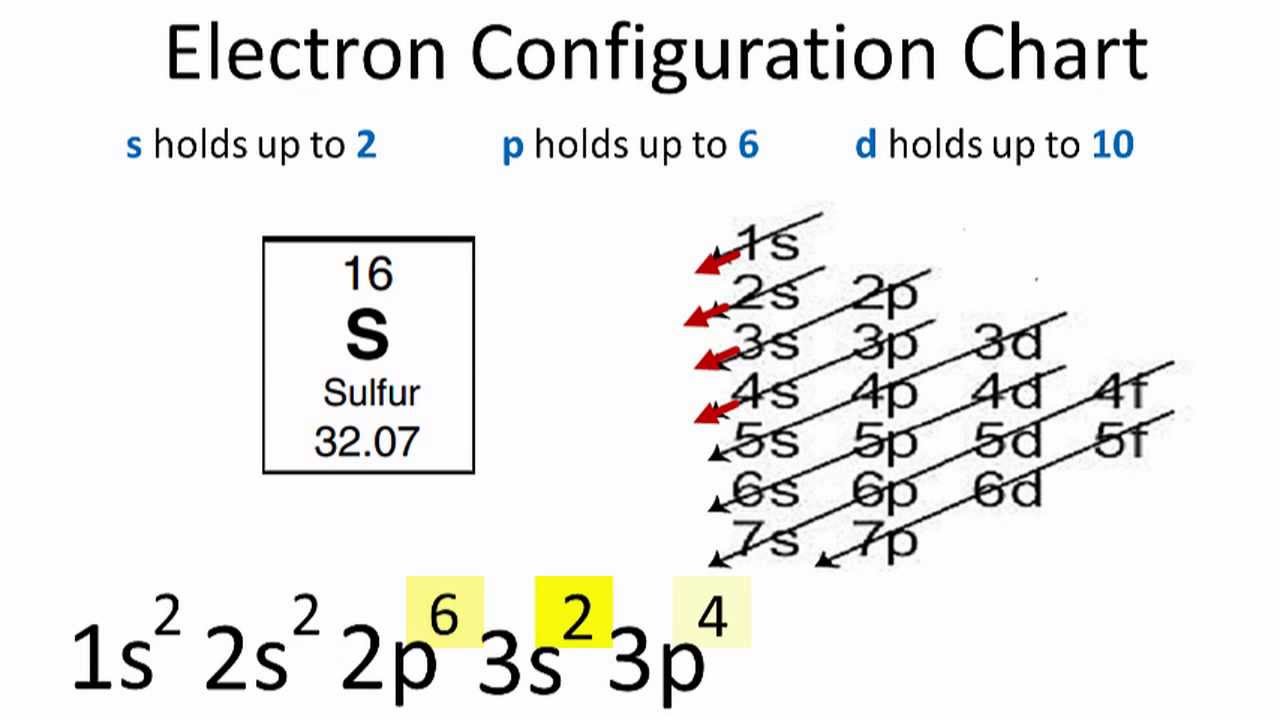
Sulfur Overview SulfurComplete Electron Configuration 1s2 2s2 2p6 3s2 3p4 Abbreviated Electron Configuration 3s2 3p4 Sources Found in pure kind and in ores choose cinnabar. Given Fxi xyj 2k and V is a closed region bounded by planes z0 z4 x0 y 0 and x y2 9 in the first octant.

Sulfur Electron Configuration Youtube
In order to write the S electron configuration we first need to know t.

. Since the 3s if now full well move to. In this question we have to write the electron configuration for sulfur atoms sulfur has 16 Electrons and 16 protons. Sulfur needs another two electrons to have a stable arrangement.
Completely dissociates to and ions. In order to write the Chlorine electron configuration we first need to know the number of. Identify the element that corresponds to the orbital diagram.
Ground state electron configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The electron configuration for sulfur is 1s2 2s2 2p6 3s2 3p4. Larissatiago7864 larissatiago7864 07102017 Chemistry High School answered Give the full electron configuration for sulfur 1 See answer larissatiago7864 is waiting for your help.
Use the electron arrangement interactive to practice building electron arrangements. University Physics Volume 3. How many total atoms are in 0330 g of P2O5.
Give the full electron configuration for sulfur Get the answers you need now. The p-orbital has three sub-orbitals. LIMITED TIME OFFER.
Each shell and subshell have a limitation on the amount of electrons that it can carry. Hichkok12 17 A covert 0330g to moles by dividing by molar mass of P2O5. Orbital can hold up to two.
Get the detailed answer. Lets call this Y. In order to write the S electron.
Chemistry 28092019 1330 desscraft30. Universidade de Sao Paulo. You must be signed in to discuss.
Give the full electron configuration for sulfur. Now the sulfide anion S2 is formed when two electrons are added to a neutral sulfur atom. I BI V x2 x2.
In this lecture we continue the discussion of Quantum Numbers and their use in. Log in Sign up. Experts are tested by Chegg as specialists in their subject area.
Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. Each sub-orbital can have a maximum of two. This means its atomic number is 16 and we can find the atomic number from the periodic table.
Home Give the Full Electron Configuration for Sulfur. Complete the electron configuration for B. Give the full electron configuration for sulfur.
Give the Full Electron Configuration for Chlorine Written By Paz Blocared Tuesday November 23 2021 Add Comment Edit. This is called quantum jump. Get the detailed answer.
The correct answer is b. Chemistry questions and answers. So well write one S.
A strong acid completely dissociates to its ions when dissolved in water. Well put six in the 2p orbital and then put the next two electrons in the 3s. Give the full electron configuration for sulfur.
In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. 2 points i Find div F. Y_Kistochka 10 8 months ago.
Give the full electron configuration for sulfur. Give the full electron configuration for sulfur. A step-by-step description of how to write the electron configuration for Sulfur S.
Write the electron configuration for iron. You might be interested in. CHEM 3311-100 Spring 2022 Recitation 1 General Chemistry Review KEY 1.
The valency of the element is determined by electron configuration in the excited state. Thus its electron configuration is. Get the answer to your homework problem.
Atoms can jump from one orbital to another orbital by excited state. Give the Full Electron Configuration for Sulfur Written By Peavy Faids1963 Sunday November 21 2021 Add Comment Edit. Give the full electron configuration for sulfur.
Try Numerade Free for 7 Days. Sulfurs has an atomic number equal to 16 which means that a neutral sulfur atom has a total of 16 electrons surrounding its nucleus. A is incorrect because does not fully.
The next six electrons will go in the 2p orbital. The electron configuration of a neutral sulfur atom will thus be. Give the full electron configuration for sulfur.
The K shell contains a 1s subshell. The subshells have a distinct shape and configuration in which the electrons move freely. Give the full electron configuration for sulfur.
The content that follows is the substance of General Chemistry Lecture 26. Sulfur is an electronegative element. Now write the electron configuration will take help from this chart right hand side first one S.
The sub-orbitals are p x p y and p z. Therefore it has 16 electrons in its outermost energy level. Give the full electron configuration for sulfur.
Give the full electron configuration for sulfur. The p orbital can hold up to six electrons. Add your answer and earn points.
GET 20 OFF GRADE YEARLY SUBSCRIPTION Pricing. Sulfurs atomic number is 16. Your answer ho 1 point ii The limits of the integral ſ.
2 points i Find div F. The maximum electrons that can be carried by the sub-shell S is 2 by P is 6 by D is 10 and the F sub-shell can carry 14. Give the full electron configuration for sulfur Nevertheless inspect the complete configuration and other exciting facts around Sulfur the most civilization dont know.
Given Fxi xyj 2k and V is a closed region bounded by planes z0 z4 x0 y 0 and x y2 9 in the first octant. Give the full electron configuration for sulfur. Explain how to get the answer clearly and youll get full stars.
He Give the full electron configuration for sulfur. This decides the electron capacity of the shells.

Find The Electron Configuration For Sulfur S And The Sulfide Ion S2 Youtube
No comments for "Give the Full Electron Configuration for Sulfur."
Post a Comment